New Hope for Early Alzheimer’s: Tau Detection Breakthrough
Alzheimer’s could be detected years before symptoms appear? Check out how tau protein testing may open doors to earlier intervention now!

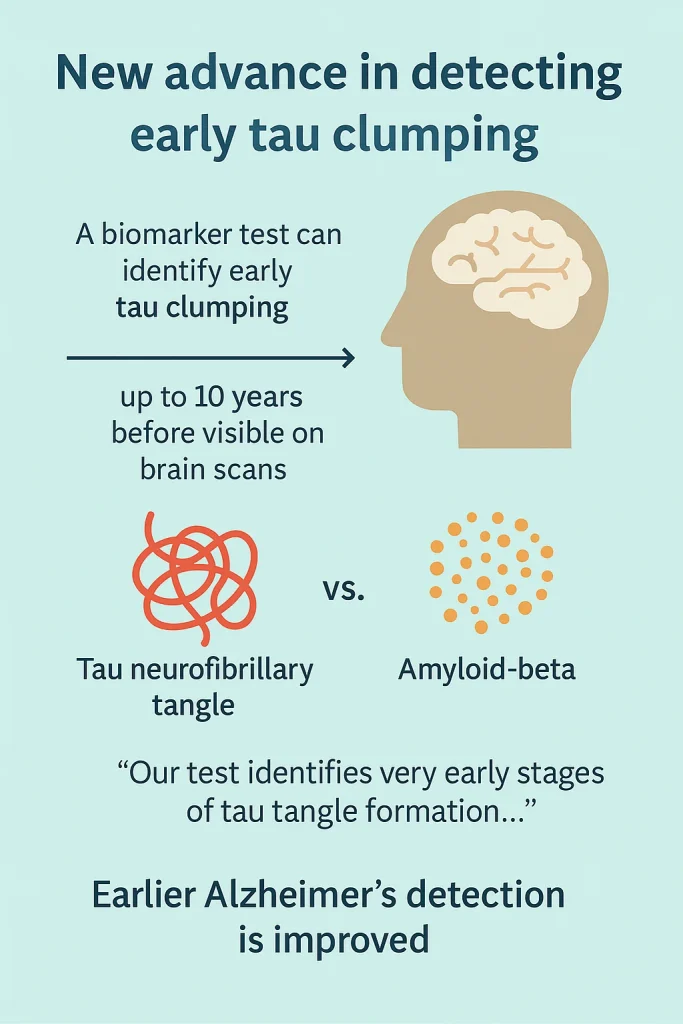
A newly developed biomarker test has shown the ability to identify early-stage tau protein clumping as much as ten years before it becomes visible through brain imaging. This advancement supports earlier detection of Alzheimer’s disease. In contrast to amyloid-beta, tau neurofibrillary tangles have been directly associated with cognitive decline.
Years in advance of visible tau tangles on brain scans, a biomarker test developed at the University of Pittsburgh School of Medicine has been shown to detect small, aggregation-prone amounts of tau protein, including its misfolded pathological forms, in the brain, cerebrospinal fluid, and possibly the blood. These findings were published in Nature Medicine.
Detection through this cerebrospinal fluid test has been linked with the severity of cognitive decline, regardless of brain amyloid presence, suggesting a promising route for earlier diagnosis and intervention.
While amyloid-beta changes often arise earlier in Alzheimer’s progression, research has indicated that tau protein clumping—forming highly ordered “neurofibrillary tangles”—serves as a stronger indicator of cognitive decline.
“Our test identifies very early stages of tau tangle formation – up to a decade before any tau clumps can show up on a brain scan.” The potential for earlier detection may increase the effectiveness of therapies, as greater benefit has been observed in those with minimal tau accumulation.
A more dependable tau biomarker continues to be sought, as many older individuals with amyloid-beta plaques never experience cognitive symptoms linked to Alzheimer’s disease. For this reason, the diagnostic guidelines established by the Alzheimer’s Association emphasize three core neuropathological elements: the combined presence of tau pathology, amyloid-beta buildup, and neurodegeneration.
Research has pointed toward the potential of early and more accessible testing methods. Previous work identified a brain-specific form of tau—BD-tau—that can be measured in blood and used to detect Alzheimer’s-related neurodegeneration. In earlier studies, it was demonstrated that certain forms of phosphorylated tau (p-tau181, p-tau217, and p-tau212) found in blood samples can signal amyloid-beta presence without relying on costly imaging procedures.
However, many of these tests primarily identify amyloid changes, leaving the early detection of tau pathology as an ongoing challenge. While tau-PET imaging remains one of the most accurate ways to measure tau levels in the brain, its use is restricted due to limited access, lower resolution, high expense, and the need for specialized expertise. In most cases, tau-PET scans detect neurofibrillary tangles only after significant buildup has occurred—by which time brain damage is advanced and far more difficult to reverse.
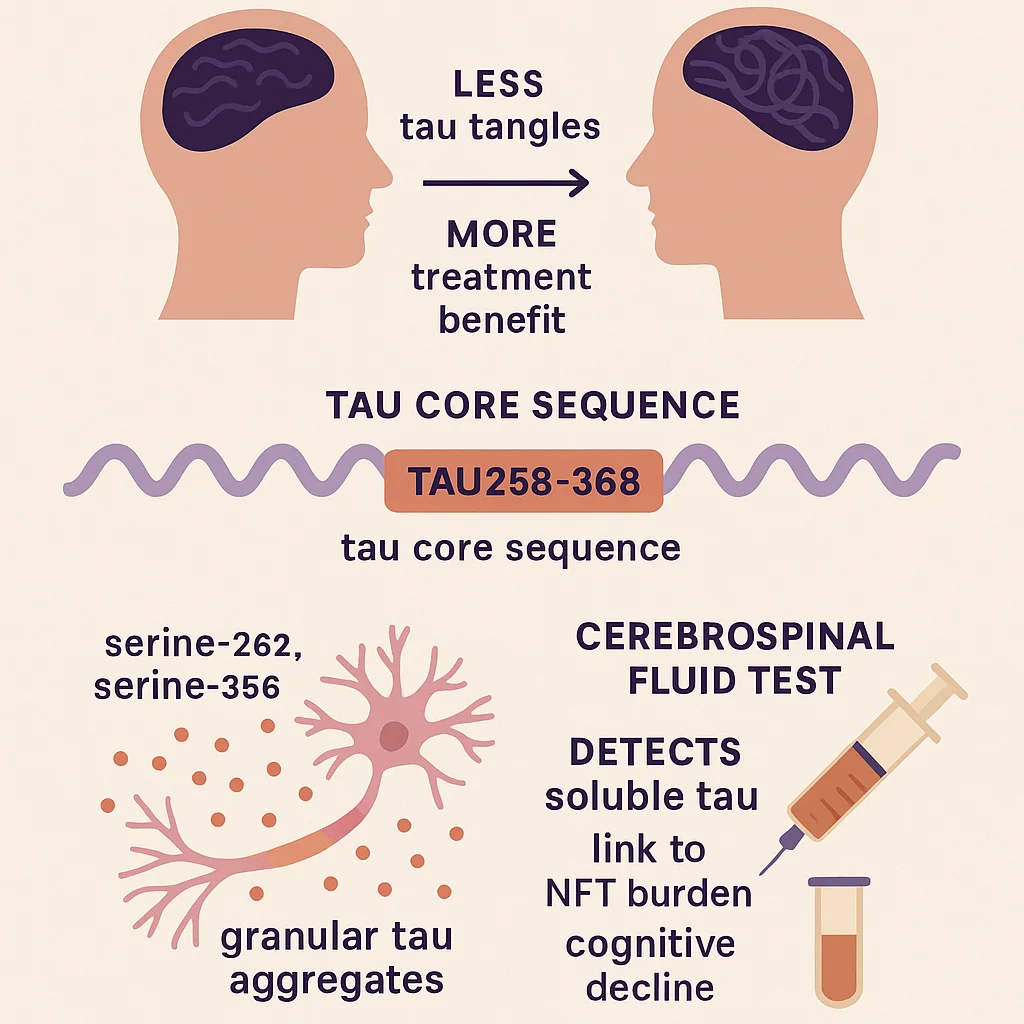
A significant advancement has been achieved in the early detection of tau-related changes linked to Alzheimer’s disease. Through biochemical and molecular methods, a research team identified a central segment of the tau protein—111 amino acids in length—essential for the development of neurofibrillary tangles. This region, referred to as tau258-368, contains sites where clumping-prone tau proteins can be detected, offering a new path toward early diagnosis and possible treatment.
Particularly, two newly identified phosphorylation sites, p-tau-262 and p-tau-356, have been found to signal early-stage tau aggregation. With timely intervention, these early tau changes may hold potential for reversal.
“Amyloid-beta is a kindling, and tau is a matchstick. A large percentage of people who have brain amyloid-beta deposits will never develop dementia. But once the tau tangles light up on a brain scan, it may be too late to put out the fire and their cognitive health can quickly deteriorate.”
Early identification of tau prone to tangling could support targeted use of emerging therapies before irreversible damage occurs.
https://www.nature.com/articles/s41591-024-03400-0
Summary
Individuals with Alzheimer’s disease who have minimal insoluble tau neurofibrillary tangles (NFTs) tend to benefit more from treatment than those with advanced NFTs. Preventing NFT formation may be possible by targeting intermediate soluble tau assemblies (STAs), though these have been poorly understood. This study identifies a tau core sequence (tau258–368) within STAs in AD brain tissue and shows that specific phosphorylation sites (serine-262 and serine-356) mark early, granular tau aggregates. A peptide based on this core alters brain activity in mouse models. A newly developed cerebrospinal fluid test detects STAs, distinguishes AD from other tau-related diseases, and correlates with tau burden and cognitive decline. These findings enhance understanding of early tau aggregation and offer potential diagnostic and therapeutic strategies for Alzheimer’s disease.
